BLV Testing
Bovine Leukosis (BLV)
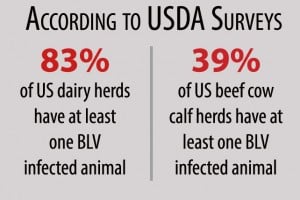
The USDA estimates that Bovine Leukemia Virus (BLV) has infected 38% of all beef herds and up to 94% of all dairy herds in the United States, with approximately 45% of all dairy cows infected. Taking action against BLV can improve overall herd health and profitability. Bovine leukosis, caused by BLV infection, is a complicated disease and eradication needs to be a multi-pronged approach. While most animals show no outward signs or symptoms of BLV, cows with advanced disease can develop lymphomas that lead to death or condemnation at slaughter. Before clinical signs, BLV suppresses the immune system, impacting cow performance and profitability by:
-
- Reducing vaccine efficacy
- Increasing risk of other diseases
- Increasing health costs
- Decreasing longevity
- Continuing to spread silently to younger unexposed animals
Sample Types
- Milk, individual (fresh, frozen or preserved)
- Serum (ELISA)
- Whole Blood
WHEN TO USE
- Test milk at dry-off or freshening (must be at least 7 days post freshening)
- Test serum by ELISA on animals over 6 months
- Test whole blood by Antelbio® STRATA-G™ BLV PCR at any age
- Animals that are within 7 days of freshening have a high probability of giving false positive results when tested because of the presence of colostral antibodies.
Testing Strategies
- Understand your herd’s risk with a BLV Herd Profile. To establish prevalence, test milk samples (DHI or hand-stripped) of the 10-most-recently-fresh cows in the first, second, third and fourth and greater lactation groups.
- The BLV Herd profile provides a starting reference point for your herd. From there, you can determine how aggressively you wish to pursue reduction.
- Test milk or blood from recently fresh or soon to be dry cows with ELISA. Testing at these stages ensures every cow is screened annually and can be managed according to her results.
- Collect whole blood samples (purple top tube) from BLV-positive cows for Antelbio® STRATA-G™ BLV PCR testing
- The Antelbio® STRATA-G™ BLV PCR test can identify susceptible cows that have become “Super Shedders,” causing the most risk to uninfected herdmates and youngstock.
- The frequency of performing this test will depend on your goal for reducing herd prevalence. It is recommended to perform the PCR test at least annually on any positive cows in the herd, but can be done after every new batch of ELISA results to expedite management decisions.
- If whole blood samples are submitted for BLV ELISA testing, they will automatically be tested with PCR if ELISA returns a positive result.
Testing Strategies
- Step 1: Establish a Baseline
Create a BLV Herd Profile to understand risk. Test milk samples of the 10 most recently fresh cows across all lactation groups (1st through 4th+). - Step 2: Annual Screening (ELISA)
Test milk or blood from fresh or soon-to-be dry cows. This ensures every cow is screened annually. - Step 3: Advanced Management (PCR)
For BLV-positive cows, use the Antelbio® STRATA-G™ PCR test. This identifies “Super Shedders” (high-risk cows).- Sample: Collect whole blood (purple top tube). Note: If blood was used for ELISA, positive results are automatically tested with PCR.
- Frequency: At least annually on positive cows, or after every ELISA batch to expedite decisions.
Result interpretation
ELISA Results
Reported as Positive, Negative or Suspect.
Antibodies against BLV were detected in the sample. Antibodies are proteins produced by the immune system in response to an exposure to Bovine Leukemia Virus.
No BLV antibodies were present in the sample.
BLV antibodies were detected in between the cutoff values and cannot be classified as positive or negative. This may occur due to early infection or low antibody concentration.
Note: A suspect result for the Milk ELISA may indicate carryover from a previous Leukosis positive cow in the milking string and should be retested with a hand-stripped sample.
PCR Results
Reported as High, Moderate, Low, or Undetected with a numeric value indicating the number of BLV copies per white blood cell. If a cow is ELISA positive but PCR negative, it means she has been exposed to BLV and has antibodies present, but the virus is undetectable or at very low levels at the time of testing.
A high concentration of BLV proviral DNA was detected.
A moderate amount of proviral DNA was detected.
A low amount of proviral DNA was detected.
No BLV proviral DNA was detected in the sample.
| Test Type Values | Positive | Negative | Suspect |
|---|---|---|---|
| Milk ELISA | >0.3 | <0.1 | 0.1 – 0.3 |
| Blood ELISA | >1.0 | <0.5 | 0.5 – 1.0 |
| Test Type Values | High | Moderate | Low | Undetected |
|---|---|---|---|---|
| STRATA-G BLV PCR PVL | >1.000 | 0.500-1.000 | < 0.500 | 0.000 |
FAQs
What is Bovine Leukosis (BLV) and how does it affect cattle?
Bovine Leukemia Virus (BLV) is a blood-borne viral infection that weakens the immune system, reduces production, and decreases herd profitability. While many infected cows show no symptoms, advanced cases can develop lymphomas (cancerous tumors) that lead to death or carcass condemnation. The USDA estimates that BLV infects up to 94% of U.S. dairy herds and 38% of beef herds. Managing BLV helps improve herd health, reproduction, and longevity.
How is BLV transmitted between cattle?
BLV spreads through blood contact, often via contaminated needles, dehorners, or ear taggers, as well as through insects or close contact with infected animals. Cows identified as “super shedders” pose the greatest risk for infecting herd mates and youngstock.
What testing options are available for BLV detection?
CentralStar offers two BLV testing methods:
- ELISA testing (milk, serum, or whole blood): Screens for antibodies against BLV.
- AntelBio™ STRATA-G™ BLV PCR (whole blood): Measures the amount of BLV DNA in the blood, categorizing cows by the shedding status.
Together, these tests help producers monitor infection prevalence and make targeted culling or management decisions.
How are BLV test results interpreted?
- ELISA Positive: Antibodies detected. The cow has been exposed; confirm shedding status with PCR.
- ELISA Suspect: Retest in 3–4 weeks or confirm with PCR.
- ELISA Negative: No antibodies detected. Retest annually
- PCR High: Cow is a super shedder with high viral load; poses greatest transmission risk.
- PCR Moderate/Low: Lower risk but still potentially infectious.
- PCR Undetected: No BLV DNA found; retest annually.
When and how should BLV testing be done?
- Milk ELISA: Test at dry-off or freshening (at least 7 days post-calving).
- Serum ELISA: For animals over 6 months of age.
- Whole Blood PCR: Test at any age to confirm BLV-positive animals and identify super shedders.
A BLV Herd Profile—testing milk from the 10 most recently fresh cows across multiple lactations—helps establish baseline prevalence and guide herd control strategies.
What does it mean when a cow is ELISA positive but PCR negative?
The cow has been exposed, has antibodies, but virus is undetectable/very low.
CentralStar’s laboratories provide sample analyses on milk, blood, fecal, and tissue samples for a variety of production, disease and health-related traits. More than 6 million samples are processed annually using state-of-the-art equipment and techniques including infrared spectroscopy, flow cytometry, ELISA, PCR, and more.
CentralStar laboratory services are intended solely for the detection of specific microorganisms or viruses in approved sample types. These services do not evaluate, certify, or guarantee the safety of milk for human consumption. It is recommended that interpretation of the results provided, and management decisions based on these results be done under the advisement of a veterinarian.